Why Does Fluorine Have a High Ionization Energy
I know you have trouble seeing that H. David Rawn in Organic Chemistry Study Guide 2015 37 Bond Dissociation Energies.
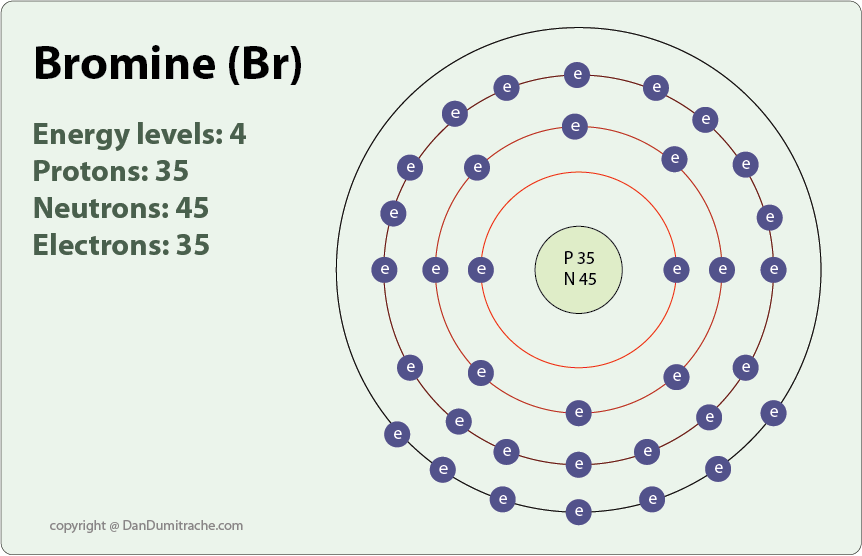
Why Does Fluorine Have A Higher Ionization Energy Than Bromine Socratic
A comprehensive database of more than 67 periodic table quizzes online test your knowledge with periodic table quiz questions.

. Note that the periodic table of elements page is provided in order to help navigate abundant chemical element data available in PubChem - each element also has a dedicated page with a lot more information available about each element including references. A Lewis base or electron-pair donor is a molecule with one or more high-energy lone pairs of electrons which can be shared with a low-energy vacant orbital in an acceptor molecule to form an adduct. Low energy easy to remove electrons.
The bond dissociation energy is the energy requiredan endothermic processto break a bond and form two atomic or molecular fragments each with one electron of the original shared pair. On the other hand the properties like Atomic radii ionic radii and metallic character decrease as we move from the left to right direction in the periodic table and increase in the top to bottom. Or especially the first electron and then here you have a high ionization energy.
FlexBook Platform FlexBook FlexLet and FlexCard are registered trademarks of CK-12 Foundation. Electronegativity ionization energy and electron affinity increase as we move from the left to right direction in the periodic table and these properties decrease as we move top to bottom. Fluorine and sometimes rare gases possess this ability as well.
When they have an noble gas configuration ions have a stable filled outer electron level. In addition to H possible electron-pair acceptors Lewis acids include neutral molecules such as BF 3 and high. So this is high high ionization energy and thats the general trend across the periodic table.
Spectroscopy does not generally refer to diffraction studies where particles such as neutrons electrons or high energy X-rays interact with a regular arrangement of molecules as in a crystal. Elements with extremely high ionization energies are incapable of transferring electrons and elements with extremely low electron affinity are incapable of absorbing electrons. The atoms of such elements tend to share electrons with atoms of other elements or atoms of the same element in such a way that both atoms achieve octet configuration in their respective.
Thus a very stable bond has a large bond dissociation energymore energy must be. Along with the increased distance of the outer electrons from the nucleus the shielding effect of the inner electrons causes ionization energy to decrease going down a column of the periodic table. Boiling Point K.
As you go from left to right you go from low ionization energy to high ionization. A low ionization energy. Our online periodic table trivia quizzes can be adapted to suit your requirements for taking some of the top periodic table quizzes.
Molecules have quantized energy levels that can be analyzed by detecting the molecules energy exchange through absorbance or emission.

Statement 1 Nitrogen Atom Has Higher Ionization Energy Than Fluorine Atom Statement 2 Youtube

Periodic Trends In Ionization Energy Ck 12 Foundation

Periodic Trends If Fluorine Has A Lower Electron Affinity Than Chlorine Why Does It Have A Higher Ionization Energy Chemistry Stack Exchange

Periodic Trends If Fluorine Has A Lower Electron Affinity Than Chlorine Why Does It Have A Higher Ionization Energy Chemistry Stack Exchange
Comments
Post a Comment